
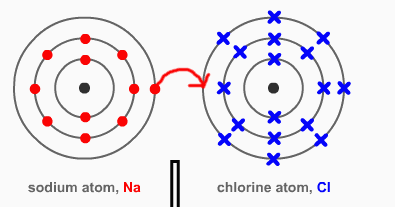
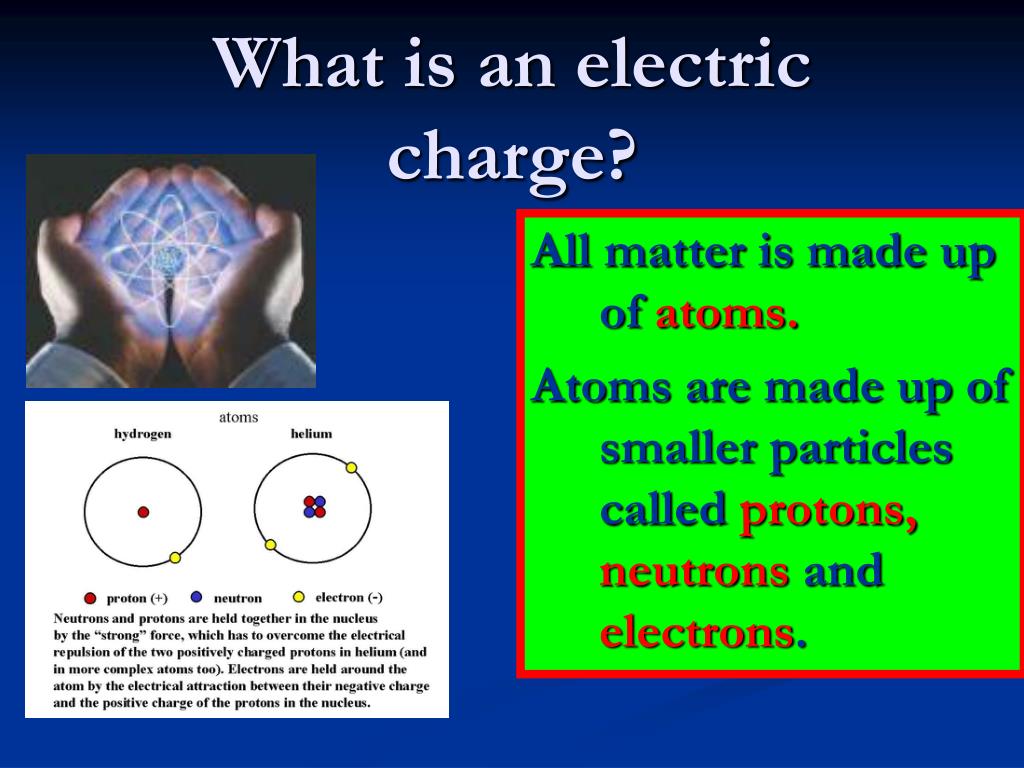
When trains carrying chlorine gas derail. Neutral chlorine atoms instantlyĬombine to form Cl 2 molecules, which are so reactive that entire communities are evacuated Into flame when it comes in contact with water. The nucleus contains positively charged particles called protons and uncharged particles called neutrons. All matter is made up of atoms, and an atom has a center, called a nucleus. Sodium metal, for example, which consists of neutral sodium atoms, bursts Negatively charged particles in an atom are known as electrons. Has an enormous impact on the chemical and physical properties of the atom. The gain or loss of electrons by an atom to form negative or positive ions By adding one moreĮlectron we get a negatively charged Cl - ion with a net charge of -1. A neutral chlorineĪtom, for example, contains 17 protons and 17 electrons. By removing an electron from this atom we get a positivelyĬharged Na + ion that has a net charge of +1.Ītoms that gain extra electrons become negatively charged. A neutral sodium atom, for example, contains 11 protonsĪnd 11 electrons. The VIA elements gain two electrons to form anions with a 2- charge. And all of them form an anion with a single negative charge. Neutral atoms can be turned into positively charged ions by removing one What makes an ions charge negative Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. When an ion is formed, the number of protons By definition,Īn ion is an electrically charged particle produced by either removing electronsįrom a neutral atom to give a positive ion or adding electrons to a neutralĪtom to give a negative ion. Since an ionic compound consists of an equal number of positive ions and negative ions, so the overall charge on an ionic compound is zero.Įxample: Sodium chloride (NaCl) is an ionic compound which is made up of equal number of positively charged sodium ions (Na +) and negatively charged chloride ions (Cl –).Atoms are neutral they contain the same number of protons as electrons. If an atom or molecule gains an electron, it. The forces which hold the ions together in an ionic compound are known as ionic bonds or electrovalent bonds. Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. In an ionic compound, the positively charged ions (cations) and negatively charged ions (anions) are held together by the strong electrostatic forces of attraction. The compounds which are made up of ions are known as ionic compounds. Those ions which are formed from groups of joined atoms are called compound ionsĮxample: Ammonium ion NH 4 +, is a compound ion which is made up of two types of atoms joined together, nitrogen and hydrogen. A negatively charged ion is known as a nion.
Positively charged ions are called cations, while. Join / Login > Class 9 > Chemistry > Atoms and Molecules > About Ions > Fill in the blanks:Negatively charged io. Question: In chemical reactions, atoms often lose or gain electrons to form charged particles called ions. Those ions which are formed from single atoms are called simple ions.Įxample: Sodium ion, Na +, is a simple ion because it is formed from a single sodium atom, Na. Click hereto get an answer to your question Fill in the blanks:Negatively charged ions are called. The ions of all the non metal elements are anions. Now, since an anion is formed by the addition of one or more electrons to an atom, therefore, an anion contains more electrons than protons. A normal atom (or a neutral atom) contains an equal number of protons and electrons. Commonly, anions and cations are called ions. An anion is formed by the gain of one or more electrons by an atom.Įxample: A chlorine atom gains 1 electron to form a chloride ion, Cl –, which is an anion.Īn anion contains more electrons than a normal atom. The key difference between anion and cation is that anions are the negatively charged ions formed from neutral atoms whereas cations are positively charged ions formed from neutral atoms. The ions of all the metal elements are cations.Ī negatively charged ion is known as anion. A cation is formed by the loss of one or more electrons by an atom.Įxample: Sodium atom loses 1 electron to form a sodium ion, Na +, which is cation : An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons.Įxample:Sodium ion Na +, magnesium ion Mg 2+, chloride ion Cl –, and oxide ion O 2–.Ī positively charged ion is known as cation. What are the Two Types of Ions and how are they DifferentĪn ion is a positively or negatively charged atom (or group of atoms).


 0 kommentar(er)
0 kommentar(er)
